

A fuel cell is an electrochemical device that utilizes hydrogen and oxygen to produce electricity. While a fuel cell operates similarly to a battery, it does not rundown or requires recharging, and it will produce electrical energy as long as fuel is supplied. Because the conversion of the fuel to energy takes place through an electrochemical process, rather than combustion, a fuel cell is cleaner and quieter than a gasoline-powered generator.
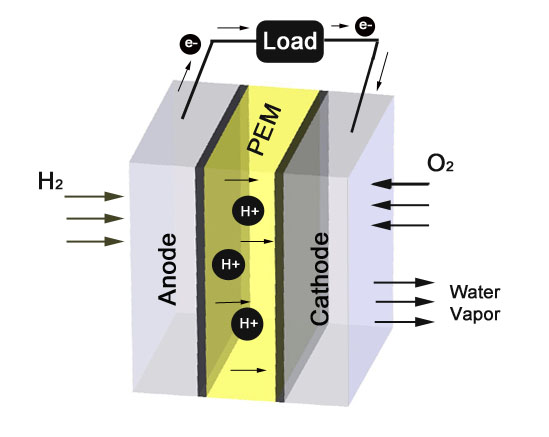
The accompanying image illustrates the operation of a Proton Exchange Membrane (PEM) fuel cell. (UltraCell uses a proprietary version of this fuel cell type). One of the most common types of fuel cells is the proton exchange membrane (PEM) fuel cell, which uses hydrogen as a fuel. It consists of a membrane that allows hydrogen protons to transfer from the anode to the cathode with catalysts on both electrodes to assist in the chemical reactions. Each membrane cell is connected to an electric circuit. Hydrogen is provided to the anode while oxygen is supplied to the cathode. The hydrogen breaks down at the anode into electrons and protons. The electrons pass through the electric circuit to provide power to a load while the protons pass through the membrane to the cathode. The electrons and protons combine with oxygen at the cathode and produce water.
 |
UltraCell XX25™ Fuel Cell |

